The Senate voted 68-31 Thursday evening to pass the omnibus spending bill that includes a rider granting the FDA authority over synthetic nicotine. The bill was passed Wednesday by the House, and will now go to President Biden to be signed into law.
The $1.5 trillion spending bill funds major parts of the government through September (the end of the 2022 fiscal year), and also appropriates $13.6 billion for aid to Ukraine. Like most such bills, it is loaded with senators’ pet projects and completely unrelated legislation—like the nicotine law.
The language in the bill will make products that use synthetic nicotine (and all forms of nicotine) subject to the same FDA rules as tobacco-derived nicotine products. The nicotine rider received no hearings or debate in either the Senate or House. The law is based on language from a House bill introduced last December, which also was not debated.
The law will take effect 30 days after Biden signs it, which will probably happen early next week. Manufacturers then have 30 additional days to submit Premarket Tobacco Applications (PMTAs) for synthetic products already on the market. Those who submit applications must receive authorization from the FDA within 60 days or remove those products from the market (or be subject to enforcement).
The law prevents marketing (after the effective date in about 30 days) of products the FDA considers synthetic nicotine variations of products with previously denied PMTAs. In other words, the manufacturer of an e-liquid that differs only in the type of nicotine from an e-liquid that has already received a Marketing Denial Order (MDO) cannot submit a new PMTA. However, new products—with different ingredients and names—can be introduced until the effective date of the law, and PMTAs for those products may be submitted.
For those concerned that this means the end of the independent vaping industry, it doesn’t—but it will hurt. Some businesses may decide to close rather than continue fighting a system stacked against them, but others have said they will continue.
“I know that it is very hard not to panic, I would encourage everyone to please remember we have been on the cusp of eradication many times before,” wrote American Vapor Manufacturers Association (AVM) President Amanda Wheeler on Facebook. “We are a resilient bunch and we will always keep fighting.”
There are a variety of legal strategies manufacturers will employ in the coming weeks to challenge the synthetic law—and many still have legal and administrative challenges pending for previously issued MDOs. Expect this to be a major stream of vaping industry news in the near future.
As we explained earlier this week, the nicotine language was inserted in the bill by a bipartisan group of House and Senate members, representing the interests of both anti-vaping groups and the tobacco industry. Juul Labs and RJ Reynolds actively lobbied for the bill, claiming it closed a “loophole” that allowed bad actors to “target youth” and escape FDA regulation.
The real reason Reynolds and Juul supported the bill (and state-level bills to ban synthetic nicotine and create registries of FDA-authorized products) is to protect their profits. Disposables sold by manufacturers like synthetic nicotine bad boys Puff Bar are major convenience store competitors for Juul Labs and Reynolds’ Vuse brand. And open-system products sold in vape shops and online continue to make up a big chunk of the vaping market.
The law will force thousands of products off the market that were launched by small e-liquid manufacturers in the wake of last year’s FDA mass-denial of PMTAs for flavored products.
The synthetic nicotine language changes the wording of the 2009 Tobacco Control Act to read, “The term ‘tobacco product’ means any product made or derived from tobacco, or containing nicotine from any source, that is intended for human consumption, including any component, part, or accessory of a tobacco product (except for raw materials other than tobacco used in manufacturing a component, part, or accessory of a tobacco product).” (New wording in bold type.)
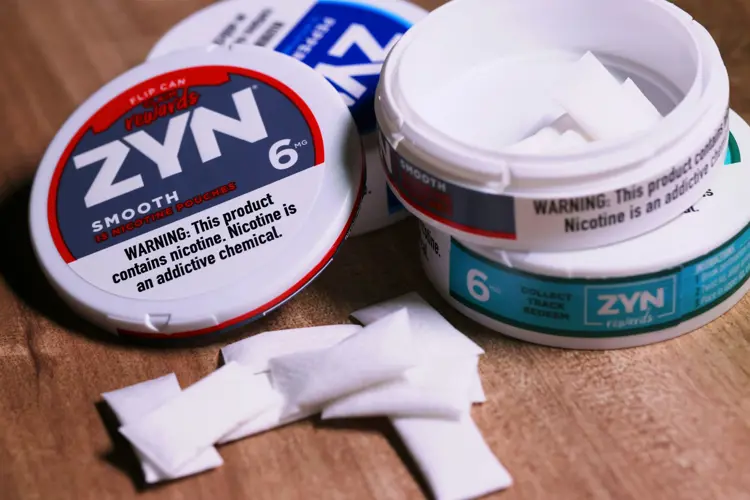
In addition to vapes, the law includes any product containing synthetic nicotine, including nicotine pouches and lozenges. The recently introduced hemp cigarettes with added synthetic nicotine will also have to leave the market, unless their manufacturer can prove they’re “appropriate for the protection of public health” (not bloody likely).
The Tobacco Control Act was originally intended to regulate tobacco—especially combustible cigarettes, which are responsible for almost all of the immense harm caused by tobacco. In 2016, the FDA’s Deeming Rule amended the Act to include vaping productsthat use tobacco-derived nicotine. Now Congress has given the agency authority over products that have no connection to tobacco at all.
The Freemax REXA PRO and REXA SMART are highly advanced pod vapes, offering seemingly endless features, beautiful touchscreens, and new DUOMAX pods.
The OXVA XLIM Pro 2 DNA is powered by a custom-made Evolv DNA chipset, offering a Replay function and dry hit protection. Read our review to find out more.
The SKE Bar is a 2 mL replaceable pod vape with a 500 mAh battery, a 1.2-ohm mesh coil, and 35 flavors to choose from in 2% nicotine.
Because of declining cigarette sales, state governments in the U.S. and countries around the world are looking to vapor products as a new source of tax revenue.
The legal age to buy e-cigarettes and other vaping products varies around the world. The United States recently changed the legal minimum sales age to 21.
A list of vaping product flavor bans and online sales bans in the United States, and sales and possession bans in other countries.