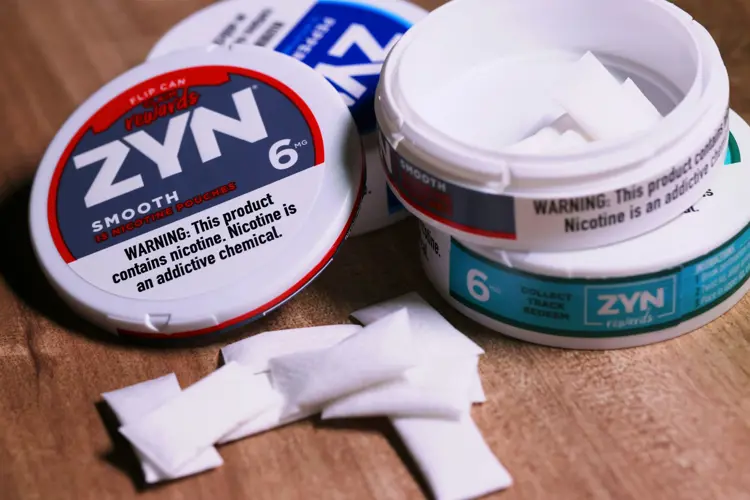
The FDA has authorized four menthol-flavored NJOY vaping products, allowing their sale in the United States. The four products are the first non-tobacco flavored vapes granted marketing permission by the agency, and the first vaping products of any kind authorized in over two years.
The products are NJOY ACE menthol pods in 2.4 and 5 percent nicotine strengths, and the NJOY DAILY Menthol (4.5 percent) and NJOY DAILY EXTRA Menthol (6 percent). The NJOY ACE device was granted marketing authorization in April 2022, along with three tobacco-flavored refill pods. The FDA also authorized tobacco-flavored versions of the two NJOY DAILY disposables in 2022.
See you in court, FDA
NJOY submitted marketing applications for the products before the September 2020 submission deadline, meaning they have been under review for about four years. Millions of flavored vaping products made by small independent manufacturers were denied review—orreceived marketing denial orders (MDOs) after the FDA conducted comical cookie-cutter reviews—within a year after the submission deadline.
“It is the responsibility of the applicant to provide the necessary evidence to obtain marketing authorization, and the FDA has made clear what’s needed to successfully achieve that outcome,” said Center for Tobacco Products (CTP) Director Brian King in a press release. “This action is further reinforcement that authorization of an e-cigarette product is possible when sufficient scientific evidence has been submitted to the agency to justify it.”
King may have to explain to a court soon what evidence NJOY provided in its premarket tobacco applications (PMTAs) that other manufacturers didn’t. The FDA has previously issued MDOs for menthol products nearly identical to the NJOY ACE and DAILY products, including menthol refills for three different authorized R.J. Reynolds Vuse devices and two authorized Logic devices.
Those MDOs have all been challenged in federal court. Logic lost its MDO appeal in the Third Circuit Court of Appeals, but has petitioned the Supreme Court to review the decision. The Reynolds cases have been consolidated and stayed by the Fifth Circuit until the Supreme Court considers (or refuses to consider) the FDA’s petition in the Triton Distribution MDO appeal
The FDA’s NJOY menthol decision was announced just a day after the Supreme Court held a conference to discuss four petitions involving challenges to the FDA’s chaotic regulation of the vaping market, including the Logic and Triton cases.
Former NJOY owners just earned a quick quarter-billion
Fun fact: when NJOY was bought by Marlboro-manufacturer Altria Group for $2.75 billion in March 2023, the purchase agreement specified that an additional $250 million would be paid to NJOY’s owners if the FDA eventually authorized the NJOY ACE menthol pod in 5 percent strength.
The FDA “authorized list” grows
For those keeping track, the four newly authorized NJOY products will be added to the FDA’s deceptive “authorized e-cigarette list,” in which the agency previously claimed to have given marketing permission to 23 “e-cigarettes and devices.”
The FDA list, however, includes a heated tobacco product and two refills (not an e-liquid-based e-cigarette), duplicate entries for one Vuse device, and another Vuse product (and its refills) no longer for sale.
With the new products added, here is the complete list of authorized e-liquid-based vapes that are currently sold in U.S. stores:
- Logic Pro + two tobacco-flavored refills
- Logic Power + one tobacco-flavored refill
- NJOY DAILY - tobacco and menthol versions
- NJOY DAILY EXTRA - tobacco and menthol versions
- NJOY ACE + three tobacco-flavored refills and two menthol refills
- Vuse Solo + two tobacco-flavored refills
- Vuse Vibe + one tobacco-flavored refill
All the products authorized by the FDA are produced by manufacturers owned by Big Tobacco companies: Logic (Japan Tobacco), NJOY (Altria), and Vuse (R.J. Reynolds).
The agency has not authorized a single product in a flavor other than tobacco or menthol, a single open-system (refillable) product, or a single bottled e-liquid. Dozens of small American manufacturers have challenged the FDA’s denial orders in federal court.
The Freemax REXA PRO and REXA SMART are highly advanced pod vapes, offering seemingly endless features, beautiful touchscreens, and new DUOMAX pods.
The OXVA XLIM Pro 2 DNA is powered by a custom-made Evolv DNA chipset, offering a Replay function and dry hit protection. Read our review to find out more.
The SKE Bar is a 2 mL replaceable pod vape with a 500 mAh battery, a 1.2-ohm mesh coil, and 35 flavors to choose from in 2% nicotine.
Because of declining cigarette sales, state governments in the U.S. and countries around the world are looking to vapor products as a new source of tax revenue.
The legal age to buy e-cigarettes and other vaping products varies around the world. The United States recently changed the legal minimum sales age to 21.
A list of vaping product flavor bans and online sales bans in the United States, and sales and possession bans in other countries.