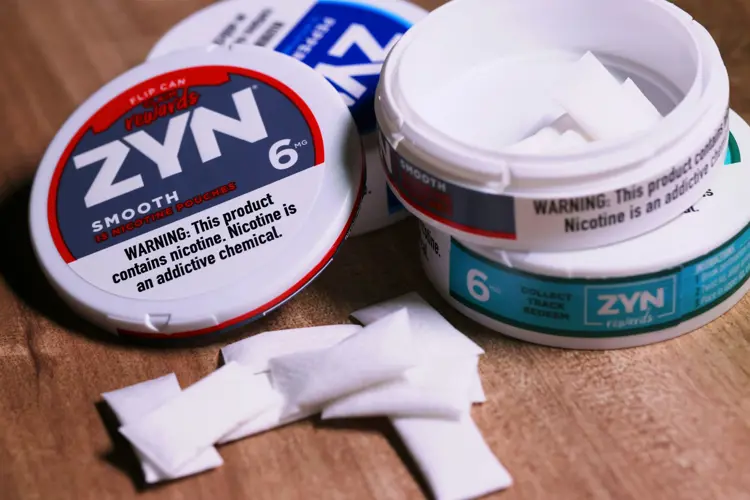
The FDA has authorized the sale of all 20 varieties of ZYN nicotine pouches, granting marketing permission to manufacturer Swedish Match, which is a division of Philip Morris International (PMI). Swedish Match submitted premarket tobacco applications (PMTAs) for the products over four years ago.
The marketing granted order (MGO) from the FDA Center for Tobacco Products (CTP) applies to the following ZYN flavors, all available in both 3 mg and 6 mg nicotine strengths:
- Chill
- Cinnamon
- Citrus
- Coffee
- Cool Mint
- Menthol
- Peppermint
- Smooth
- Spearmint
- Wintergreen
The MGO only applies to the products named in today's FDA announcement, which are all the ZYN products currently sold in the United States. If Swedish Match wants to market additional flavors, or different nicotine strengths, the company would have to submit new PMTAs and wait for new authorizations before selling the products. (There may be pending PMTAs for products not currently on the market.)
ZYN becomes the first brand of nicotine pouches to receive marketing authorization from the FDA—and the first nicotine product of any kind to receive a green light from the FDA in flavors other than mint or menthol. No vaping product in a flavor other than tobacco or menthol has received authorization.
Youth use of nicotine pouches has been low—under two percent past-30 day use last year, according to the National Youth Tobacco Survey—but the ZYN authorization came with an FDA warning to PMI: prevent youth adoption or else. The FDA can rescind authorizations if it decides youth uptake is out of hand.
“It’s critical that the manufacturer market these products responsibly to prevent youth use,” said CTP Director Brian King. “While current data show that youth use remains low, the FDA is closely monitoring the marketplace and is committed to taking action, as appropriate, to best protect public health.”
The authorization confirms that the FDA considers ZYN “appropriate for the protection of public health”—the standard for approval of new products Congress set forth in the 2009 Tobacco Control Act. Nicotine pouches and vapes were not mentioned in that legislation, but the FDA was granted the ability to seize regulatory authority over new nicotine products, which the agency did with the 2016 Deeming Rule.
ZYN arrived in U.S. test markets in 2016. Since 2020, the product has been the fastest-growing brand in the nicotine marketplace, and nicotine pouches the fastest-growing category. In 2023, ZYN held 70 percent of the $8.6 billion U.S. nicotine pouch market.
Last year, Swedish Match announced the construction of a second U.S. production facility in Colorado to meet retail demand.
Along with its popularity has come opposition. Tobacco control activist groups like Truth Initiative and the Campaign for Tobacco-Free Kids haveincluded nicotine pouches with vaping in their anti-nicotine product propaganda. Senator Chuck Schumer (D-NY), a longtime opponent of smoking and vaping, held a press conference last year to denounce ZYN, which he called "a pouch packed with problems."
Some U.S. states have already banned flavored nicotine products, including ZYN. Tobacco control groups, which work constantly to advance such bans in state legislatures and city councils, are certain to be alarmed by the ZYN authorization, and redouble their efforts.
The Freemax REXA PRO and REXA SMART are highly advanced pod vapes, offering seemingly endless features, beautiful touchscreens, and new DUOMAX pods.
The OXVA XLIM Pro 2 DNA is powered by a custom-made Evolv DNA chipset, offering a Replay function and dry hit protection. Read our review to find out more.
The SKE Bar is a 2 mL replaceable pod vape with a 500 mAh battery, a 1.2-ohm mesh coil, and 35 flavors to choose from in 2% nicotine.
Because of declining cigarette sales, state governments in the U.S. and countries around the world are looking to vapor products as a new source of tax revenue.
The legal age to buy e-cigarettes and other vaping products varies around the world. The United States recently changed the legal minimum sales age to 21.
A list of vaping product flavor bans and online sales bans in the United States, and sales and possession bans in other countries.