President Trump promised during his election campaign to “save vaping," but his administration has undermined that goal at every turn.
European Union leadership is proposing first-time minimum taxes on low-risk nicotine products, including vapes, nicotine pouches and heated tobacco.
The FDA has granted authorization for Juul Labs to market its JUUL vape device and refill pods in tobacco and menthol flavors.
SKE announces the launch of its latest prefilled pod system, the SKE CL2000.
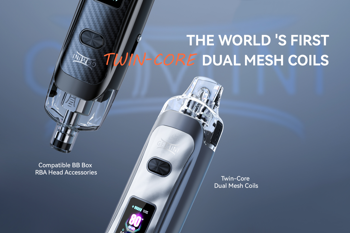
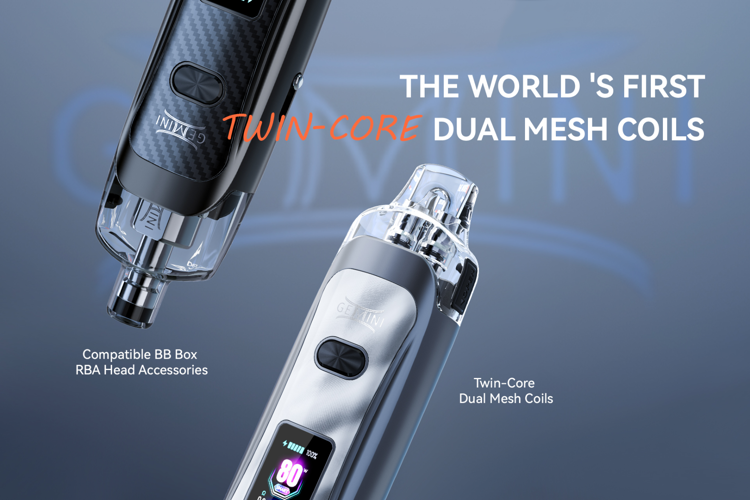
Vandy Vape releases the Gemini, an 80-watt pod vape featuring the world’s first twin-core dual mesh coil system.
Vaping360 3.0 is here with a fresh design, an expanded product database, and various improvements all over. And this is just the beginning—new updates and features are on the way!
Sort by:

The FDA still has not produced a list of legally marketed vaping products that have been submitted for PMTA consideration by the agency.

A patent dispute between two tobacco giants could end with a trade court removing PMI’s controversial IQOS device from the U.S. market.

The city of Chicago has accused an online vaping retailer of selling banned flavored products to minors without using proper age verification.

The Department of Agriculture’s final Hemp Rule, which takes effect March 22, provides regulations for farmers and CBD processors.
Explore our curated selection of the latest press releases from leading brands. Stay informed and discover the newest updates in the industry.

Following a similar decision by shipper Fedex, UPS has announced it will no longer deliver vaping products after April 5th.

The FDA has issued its final Premarket Tobacco Application rule—-four months after the deadline for manufacturers to submit applications.

The Kansas, Maryland, and North Dakota legislatures are considering bills that would restrict or tax vaping products.

The FDA has issued the first warning letters to small e-liquid manufacturers selling products without first submitting a PMTA.
President Trump promised during his election campaign to “save vaping," but his administration has undermined that goal at every turn.
The U.S. disposable vape market has grown to $2 billion in annual sales, although nearly none of the products are authorized by the FDA.
More than 30 bills that would impose severe restrictions vaping consumers’ product choices remain active in U.S. state legislatures.
The Freemax REXA PRO and REXA SMART are highly advanced pod vapes, offering seemingly endless features, beautiful touchscreens, and new DUOMAX pods.
The OXVA XLIM Pro 2 DNA is powered by a custom-made Evolv DNA chipset, offering a Replay function and dry hit protection. Read our review to find out more.
The SKE Bar is a 2 mL replaceable pod vape with a 500 mAh battery, a 1.2-ohm mesh coil, and 35 flavors to choose from in 2% nicotine.
Because of declining cigarette sales, state governments in the U.S. and countries around the world are looking to vapor products as a new source of tax revenue.
The legal age to buy e-cigarettes and other vaping products varies around the world. The United States recently changed the legal minimum sales age to 21.
A list of vaping product flavor bans and online sales bans in the United States, and sales and possession bans in other countries.
Al FAKHER announces the release of their latest replaceable pod device, the AF 30K Hypermax Advanced, in Europe.
LOST MARY unveils the Ultrasonic 35K in the U.S., the brand’s first product powered by ultrasonic atomization technology.
VOOPOO releases the VMATE PRO2, a pod vape that introduces an original intelligent ECO Mode which analyzes the user’s daily vaping habits and automatically matches the most suitable power output.
Empowering Vapers Worldwide
As a recognized authority in the vaping world, Vaping360 draws over a million monthly visitors to read our in-depth reviews and guides, breaking news, and insights on the latest vaping products and events impacting the vaping world. Our mission is to achieve a healthier, smoke-free world.